What is Nuclear Medicine?
Getting started with our product is a breeze, thanks to our well-structured and comprehensive onboarding process.
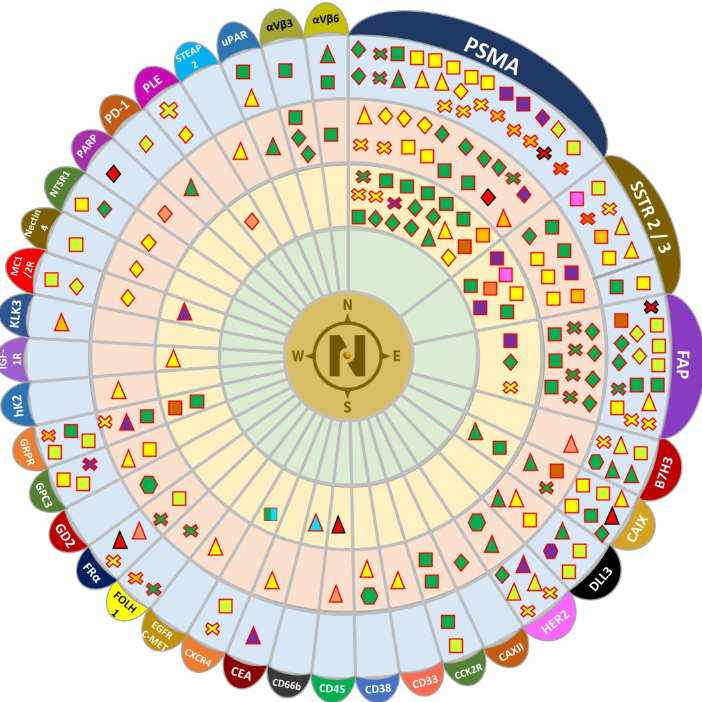
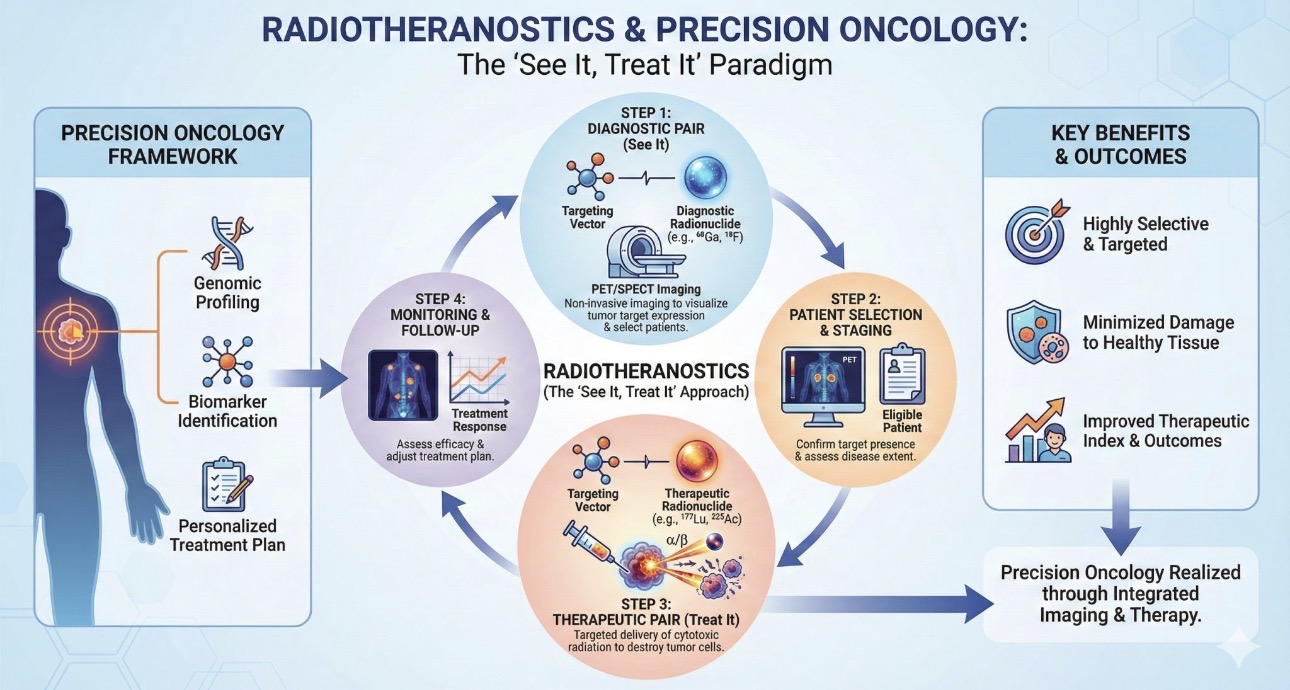
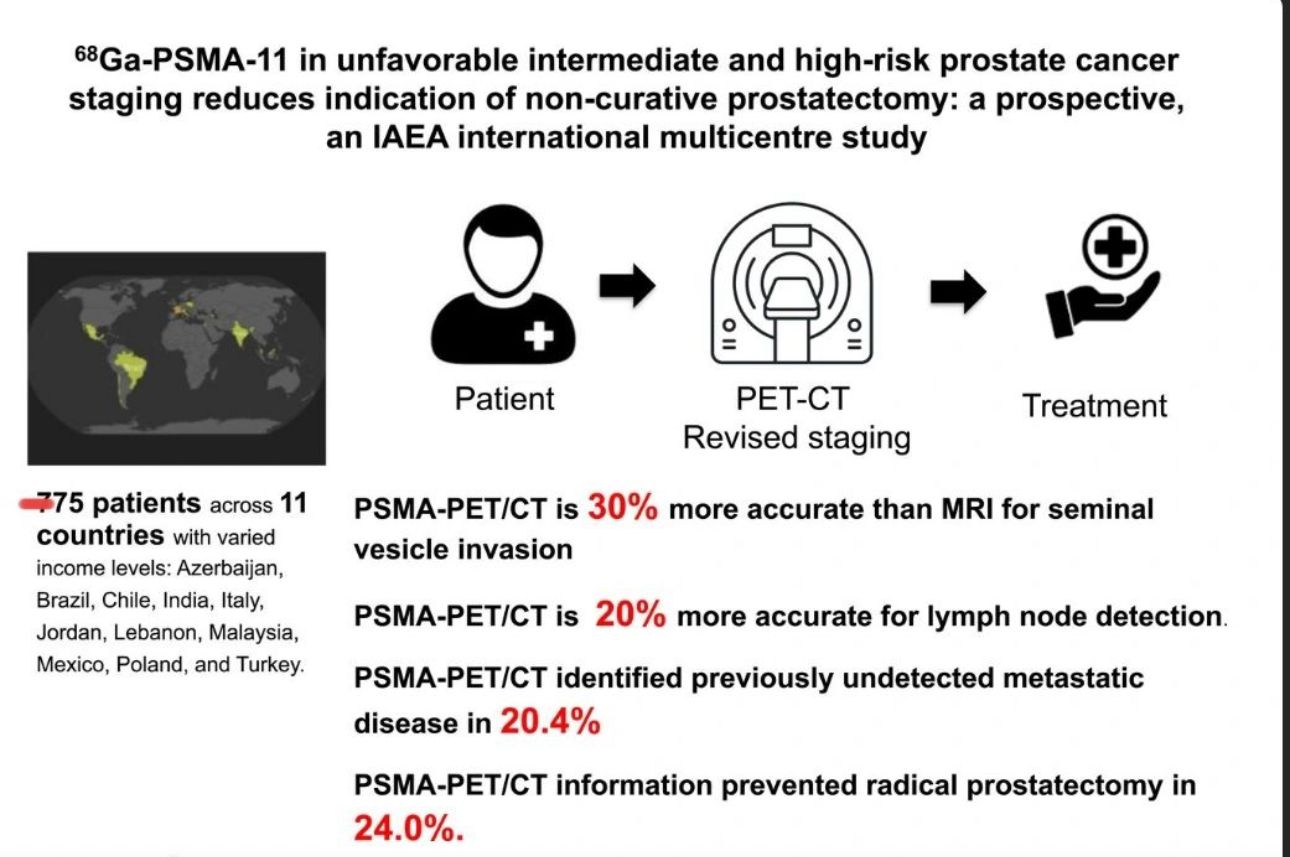
Nuclear Medicine is where advanced science meets precision healthcare.
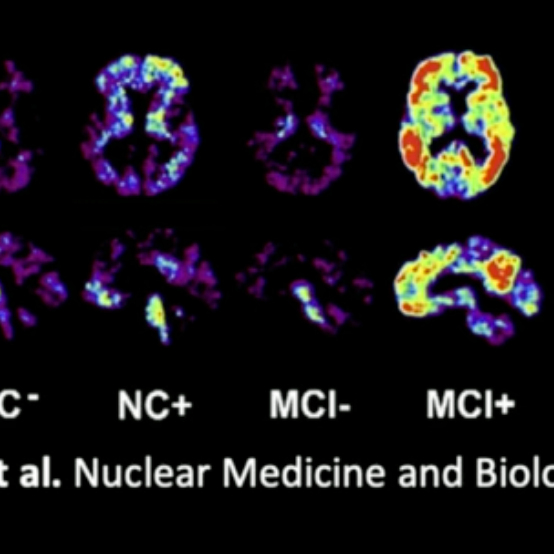
By using safe, trace amounts of radioactive materials—called radiotracers—this cutting-edge technology allows doctors to see what’s happening inside the body at the molecular level, long before structural changes become visible through conventional imaging.
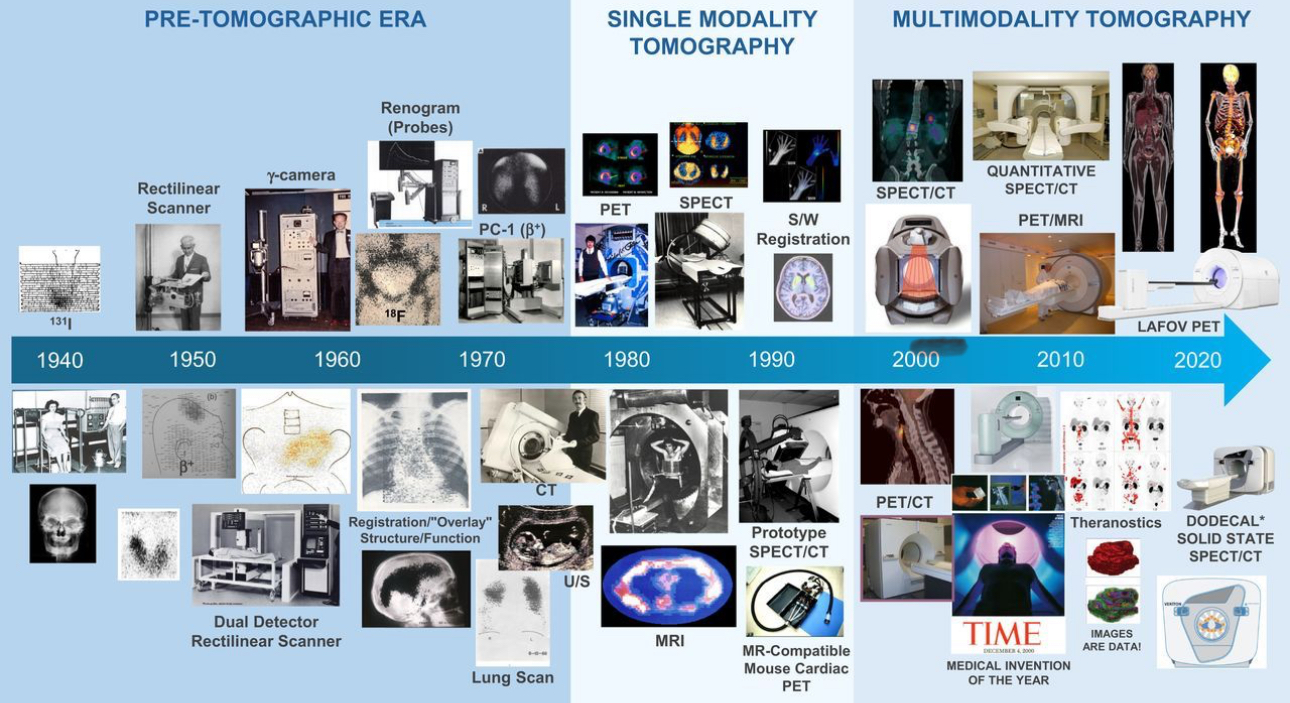
Unlike standard scans that show anatomy, Nuclear Medicine reveals function — helping detect disease earlier, guide personalized treatment, and monitor therapy effectiveness with unmatched accuracy.
From endocrinology and cardiology to neurology and oncology, nuclear medicine is transforming diagnosis, patient's management and therapeutic care across multiple specialties.
At KAX radiopharmaceutical consulting, we offer expert clinical insight to support deep understanding of the dynamic environment of innovative radio pharmaceuticals, along with the more traditional imaging!