Laura Ravasi
I am an Italian nuclear medicine physician-researcher turned executive, with roles of increasing responsibility in clinical development and medical affairs across clinical, pre-clinical, academic and pharma sectors. I provided scientific, technical, strategic & operational advice to Advanced Accelerator Applications (AAA), the start-up issued from CERN that was granted market authorisation for the first ever radiopharmaceutical drug to treat GEP-NET patients, both in the USA and EMEA: the 177Lu-DOTATATE, commercialized as Lutathera®
Education
Back in the 90’, in Milan, Italy, during my 4th year of medical school, a professor returning from the Brookhaven National Laboratories presented his own PET-brain image! Fascinated by such non-invasive new methodology, I sought ways to deepening my knowledge in this area. I joined a Fogarty fellowship at the National Institutes of Health, Bethesda, MD, under the supervision of two amazing mentors, a chemist, Prof. W.C. Eckelman and a physiologist, Prof. L. Sokoloff. I participated to many projects including building a small animal PET; assessing in vitro, in vivo and ex vivo bio-distribution of new tracers produced in house; validating quantitative and qualitative methods for image analysis; assessing methods of reproducibility of results and interpret pre-clinical data to translate into potential clinical application. Meanwhile, I was learning the basics of nuclear medicine, which I applied and practiced daily, during my following years of residency, spent in Milan, Italy at the European Institute of Oncology (IEO), under Prof. G. Paganelli, who introduced me to the therapeutic potential of nuclear medicine (radiolabeled treatments in breast, uterus, brain and neuroendocrine tumors).
Nuclear Medicine Specialist
I moved to Paris, France to work as a Nuclear Medicine specialist at the Commissariat à l’Energie Atomique (CEA). Shortly after, I seized the opportunity of being part of a research unit based in Lille, France, combining two nuclear medicine departments, a potential cyclotron and a potential pre-clinical imaging facility. Indeed, I set up and managed the MicroPET/CT, conceiving translational protocols, analyzing data and promoting the usefulness of pre-clinical imaging. Unfortunately, the radiotracers were limited for the cyclotron project never started.
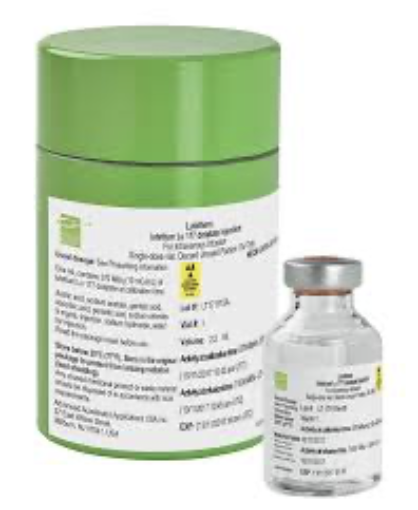
Lutathera breakthrough
In 2016, I was offered to help with the Netter-1 data in view of the FDA filing for market authorisation in the USA. I took this opportunity to move to Geneva and have worked for Advanced Accelerator Applications since then, getting Lutathera® and its diagnostic pair (NetSPOT® and SOMAKit®) launched in USA, in Europe and then expanding to Asia.
At AAA, I covered many tasks, including:
- regulatory and clinical strategy for products in early to late clinical development, including meetings with agencies and authorities;
- due diligence of tracers or biotechs for investment potential (assessing scientific, clinical and pre-clinical validity and suitability);
- strategies & plans to increase scientific awareness of the program, build key opinion leader relationships and champions, and advisory board activities;
- publication strategy, and support the development of presentation and publication materials;
- review and support investigator-initiated trials;
- building a medical affairs team and processes, including implementing SOPs to meet GCPs, for a new product launch with product launch strategy & pre-launch, launch and post-launch activities.

Local Producers
As we use local growers, our flowers last longer and smell better. Just like the event you want to celebrate with flowers!

Bio Flowers
Don't offer pesticides to your beloved one! By choosing our flowers, we guarantee you organic flowers.

Eco-Friendly Packaging
We use 100% recycled materials. We believe the best gifts should also do good for the planet.
KAX Consulting
In 2020, the Covid-19 crisis hit Mother Earth and then, it all started shaping up …
KAX was founded based on:
- Respect for our physical and mental health, for our nature, for our privacy
- Humbleness and curiosity
- Integrity and scientific approach
- Balance and innovation





